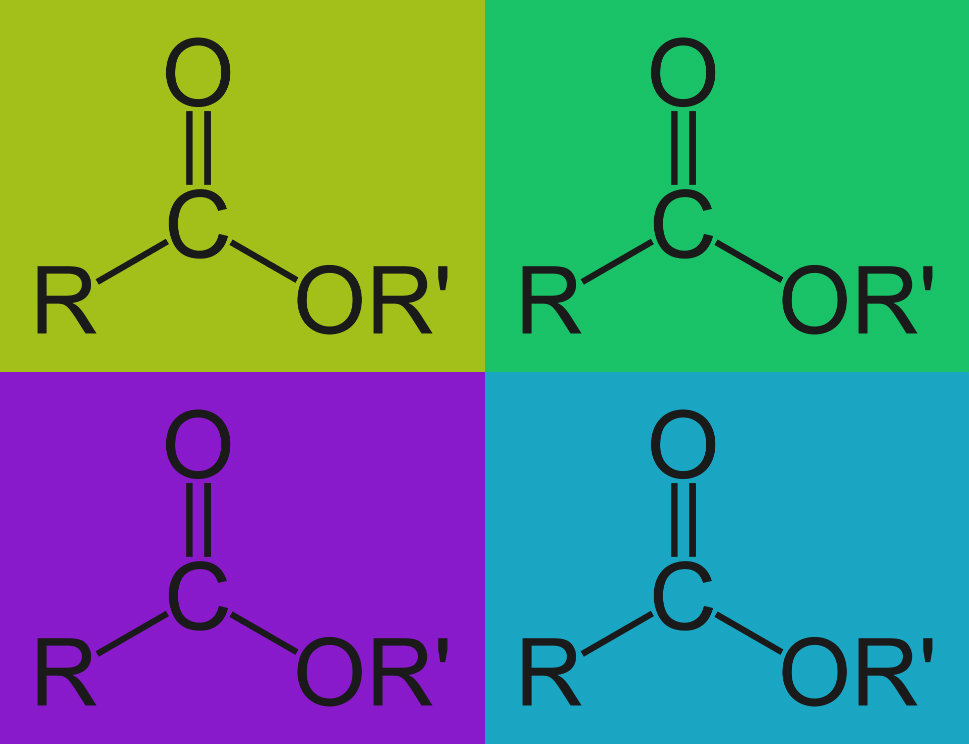
Ingredients to cause alkaline hydrolysis are commonly used in detergent formulation. Alkaline hydrolysis is a chemical reaction that takes place when tough organic residues are exposed to an alkaline solution like many of our Alconox, Inc. detergents. Alkaline hydrolysis breaks these residues into smaller more water soluble and more easily emulsified molecules.
There are two common types of residues, specifically, that are broken down by alkaline detergents. They are called esters and amides. The hydroxide ions in the alkaline solution bond to the residue, and essentially break it down through a process called nucleophilic substitution. The hydroxide ions break the esters down into carboxylic acids and alcohols. They similarly break the amides down into carboxylic acids and amines.
Esters and amides that are broken into carboxylic acids, alcohols and amines, are now smaller polar molecules and, as such, they are more easily dissolved or emulsified. More simply stated: more easily “grabbed and carried away” by an aqueous detergent during cleaning.
Many biological compounds are esters or amides, so alkaline hydrolysis is an effective pre-soaking technique with biological residues. This is why our clients in the medical device, healthcare, food and beverage, pharmaceutical, and biotech industries use alkaline detergents for their potent ability to emulsify organic and oily residues.
If needed, parts or equipment contaminated by tough or oily organic residue will be cleaned or pre-soaked in an alkaline detergent like Detonox® Ultimate Precision Cleaner (although a pre-soak in anything including Liquinox® Critical Cleaning Liquid Detergent, Alconox® Powdered Precision Cleaner or just plain water is far better than no pre-soak). This will then be followed by a thorough cleaning with Tergazyme® Enzyme Active Powder Detergent to get a bioresidue free surface (or whatever apropos Alconox Inc. detergent is called for in the next step.)
Note that in the special case where the ester is made from a C6-22 chain length organic acid, the resulting salt will be a soap. These soaps are almost always water soluble and additionally they are effective at emulsifying the esters and related compounds. So in effect alkaline hydrolysis can make emulsifying soaps in situ. A little more about detergent vs. soap.
When formulating detergents for automated spray clean-in-place, dishwasher, or cabinet washer use, using ingredients that can cause alkaline hydrolysis are very helpful. Alkaline hydrolysis is a rapid mechanism that can happen during the short period of time that a droplet of cleaning solution is on a surface before the next droplet comes along and sweeps it away. Low foaming detergents which are used in automated spray cleaning (washers and CIP systems) often rely on alkaline hydrolysis and other mechanisms to ensure effective residue removal.
To request an Alconox Inc. detergent for free, please complete the questionnaire at Get Sample. For more information about any one of our Alconox Inc. detergents, consult the technical bulletin for each product. Or click here to access each of our detergent’s Safety Data Sheets.
Do you have a critical cleaning question for the experts at Alconox Inc.? Search TechNotes to see if it’s been answered before or Ask Alconox.



