Join Alconox Inc. at MD&M West In Anaheim – February 6-8, 2024
Join Alconox Inc. at MD&M West In Anaheim – February 6-8, 2024 We are really excited to be heading to Anaheim California for MD&M West. Our cleaning experts will be on-hand to answer all your medical device cleaning questions. Plus,…
Cleaning Tissue Culture Flasks
Q: What is the recommended detergent for cleaning tissue culture flasks?
A: When it comes to maintaining a sterile and contamination-free environment in tissue culture labs, proper cleaning of tissue culture flasks is paramount. The task is not just about cleanliness; it’s about ensuring that there’s no residue left behind that could potentially impact the growth and health of cell cultures.
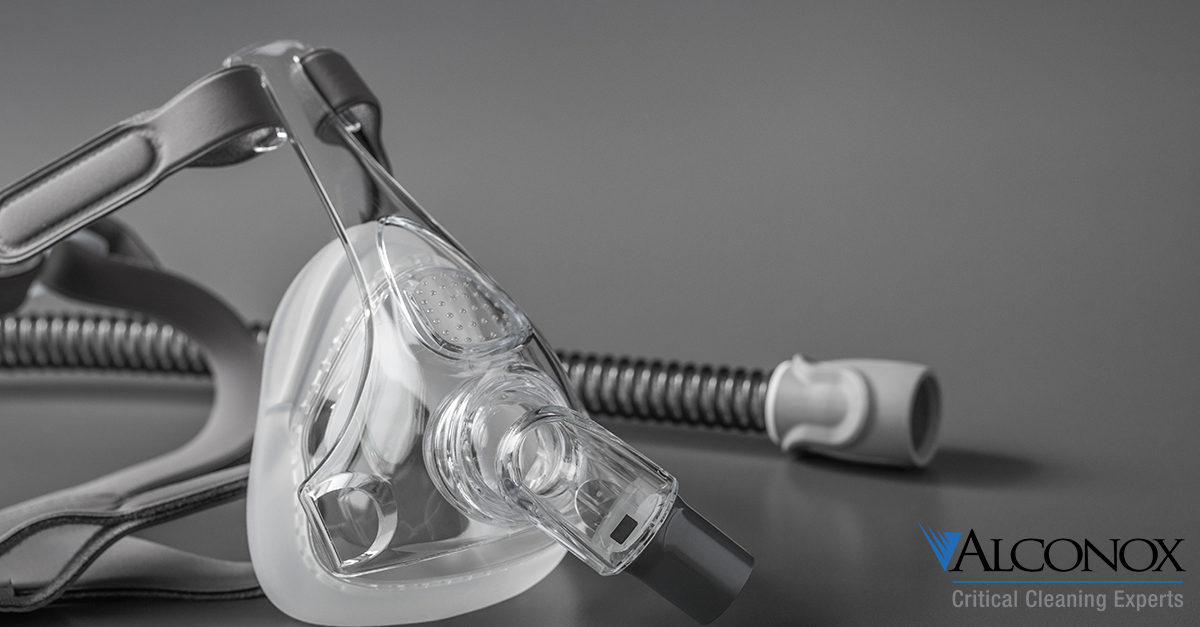
CPAP Mask Manual Cleaning
Q. What do you recommend for cleaning of CPAP masks manually? In the directions from the mask manufacturer we use at our facility it says to clean with ‘Alconox’ so I assume the powdered precision cleaner? A. Continuous positive airway…
See You At PACK EXPO in Las Vegas – September 11-13, 2023
PACK EXPO is happening September 11-13 in Las Vegas. Reconnect at PACK EXPO Las Vegas and Healthcare Packaging EXPO and experience the latest innovations to move your business forward. We are at booth SL-6920. Come stop by our booth to…
Remove Endotoxin Residue
Q: Does Alconox detergent remove endotoxin?
A: Detergents like Alconox® Powdered Precision Cleaner and Liquinox® Critical Cleaning Liquid Detergent, and others, have been used for decades around the globe to create surfaces devoid of residues. A detergent cleaning is a vital part of a depyrogenation program.