Q. Thank you for your assistance with our cleaning questions. We have seen a statement in literature that we wanted to ask more about. What does “detergent is the last to leave the equipment surface” or “last to rinse” mean? This seems like a broad statement, can it be supported by literature/documentation?
A. A likely source of a literature use of “detergent last to rinse” is based on the physical behavior of surface active agents and the interchangeable use of the word detergent and surfactant in common usage. More properly one should say the surfactant is the last to rinse.
Surfactants or surface active agents are made of molecules that have one end that is hydrophilic (water loving) and the other end is hydrophobic (water hating). Surface active agents in aqueous solutions are attracted to solution surface interfaces because the hydrophobic end of the surfactant molecule is repelled from the bulk water solution while the hydrophilic end of the molecule is attracted to the water solution. In theory, the surfactant is most stable when arranged in a film.
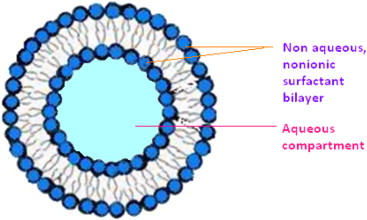
The film’s structure is made up of one side where the hydrophobic tails are facing outwards towards the solution/surface interface and the hydrophilic ends of the molecules are facing inward towards the water solution. By increasing the concentration of surfactants by adding detergent to water, a monolayer surfactant film will form in the solution until a critical micelle concentration is achieved. Micelles are balls of surfactants arranged with their hydrophobic ends facing inwards and their hydrophilic ends facing outwards. Micelles are responsible for emulsifying because the inner regions of micelles can hold hydrophobic oily molecules emulsified in the water solution.
Cleaning is typically done with surfactant concentrations above the critical micelle concentration. As the cleaning solution is rinsed away, you drop below the critical micelle concentration of surfactant, promoting mass displacement of the micelles as well as the remaining surfactant molecules (either individual or monolayer) by rinsing. Continued rinsing further dilutes the monolayer and removal of the surfactants, thereby essentially being the last molecules from the cleaning process to rinse away. Hence, the “last to rinse” statement appearing. And is also why many cleaning validation programs across multiple regulated industries around the globe use specific detection of the surfactant as the residue detection method. (Non specific methods like TOC and conductivity will also of course detect residual surfactant.)
With the limits of quantitation in the analytical methods used for cleaning validation studies and successive rinse studies, Alconox Inc. technical support is not aware of detecting different rates of rinsing among highly water soluble detergent ingredients. As a practical matter with highly water soluble detergent ingredients, all ingredients seem to rinse at the same rate in gross dip rinsing studies.
Feel free to contact us any time with further questions about your specific cleaning, cleaning validation, and rinsing needs.
To request any Alconox Inc. detergents for free, please complete the questionnaire at Get Sample. For more information about any one of our Alconox Inc. detergents, consult the technical bulletin for each product. Or click here to access each of our detergent’s Safety Data Sheets.
Do you have a critical cleaning question for the experts at Alconox Inc.? Search TechNotes to see if it’s been answered before or Ask Alconox.



