Using Alconox to clean Rexolite
Q. Is Alconox okay to use on Rexolite? A. According to http://www.rexo
Is there an equivalent to Xylene in the Alconox product line?
Q. Currently we are using Xylene to clean equipment that is used with extremely non-polar compounds. Does Alconox have a cleaning product that would be equivalent to Xylene but not as harmful and dangerous to work with? A. Even extremely non-polar com
What are clean in place (CIP) standard operating procedures (SOPs)?
Q. What is clean in place (CIP)? What are four reasons the pharmaceutical industry commonly employs clean in place (CIP) systems? Does Alconox, Inc have information on CIP Standard Operation Procedures (SOPs)? A. CIP stands for Clean-In-Plac
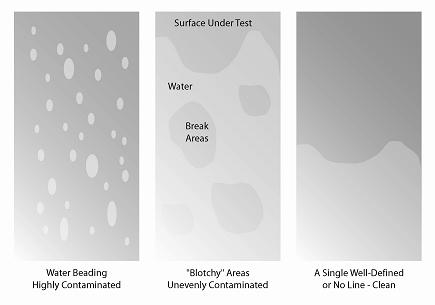
What is the water break test?
The water break test, a fairly crude test, is suitable for detecting films of process oils and heavy fingerprints. It does not readily detect non-hydrophobic residues. This test is often used for parts washing and may not be suitable for precision cleaning applications.
Is Liquinox filtered?
Q: When bottling is Liquinox filtered? A: Liquinox, as sold in the standard package, is filtered to 25 microns, but packaged in new but unwashed containers in an open air reasonably clean liquid detergent factory that is certainly not a clean…