Advanced Cleaning Mechanisms: Alconox Powder in an Auto Dispenser
Q: Looking to implement an auto dispenser for our Alconox, and it is a little bit of a struggle. Are you aware of any test equipment, or alternative methods of measuring which may be able to continuously measure the pH/conductivity at…
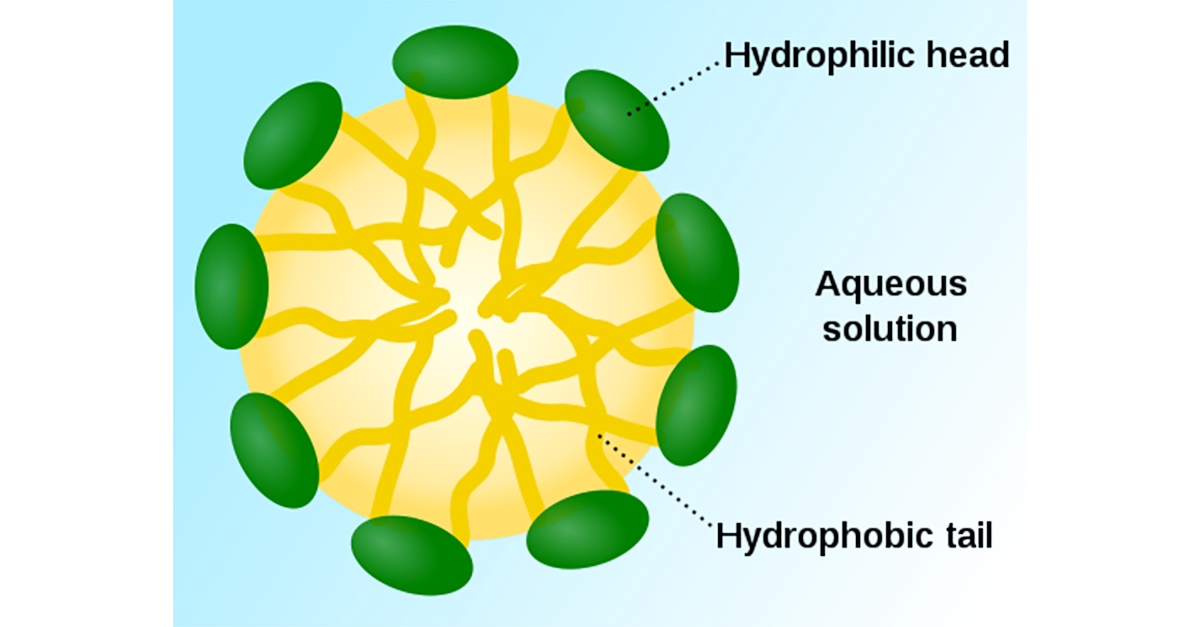
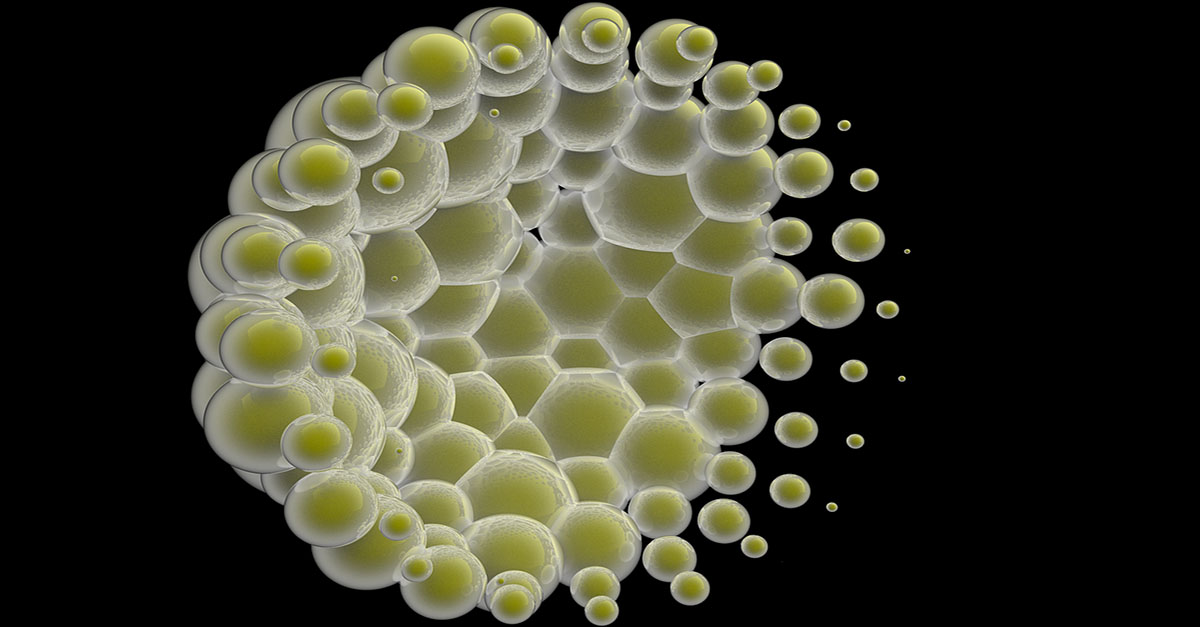
Advanced Cleaning Mechanisms: The Critical Micelle Concentration of Detergent
The critical micelle concentration is the minimum concentration at which the emulsifiers in a detergent will coalesce in to membrane structures of globes, rods or sheets with their hydrophobic (water hating) ends on the inside of the membrane and their hydrophilic (water loving) ends on the outside such that hydrophobic oily residues can be emulsified inside these membrane structures. Micelles are further elaborated on below. If you are below the critical micelle concentration, you do not have effective emulsifying of oily residues.
Advanced Cleaning Mechanisms: The Last to Rinse
Q. What does “detergent is the last to leave the equipment surface” after rinsing mean? This seems like a broad statement, can it be supported by literature/documentation?
A. The only source of “detergent last to rinse” that I am aware of is based on the physical behavior of surface active agents and the interchangeable use of the word detergent and surfactant in common usage. More properly you should say the surfactant is the last to rinse. Learn More about Surfactant.
Advanced Cleaning Mechanisms: Cold or Warm for Proteins?
Q: Many of your Tergazyme related TechNotes and recommendations include warm temperatures. Warm temperatures can still denature some hormones and proteins thus causing cleaning problems. I would probably start with a cool (room temp or cooler) rinse (water). Then move to the 1-2% cleaning solution in warmer temperatures. Agreed?
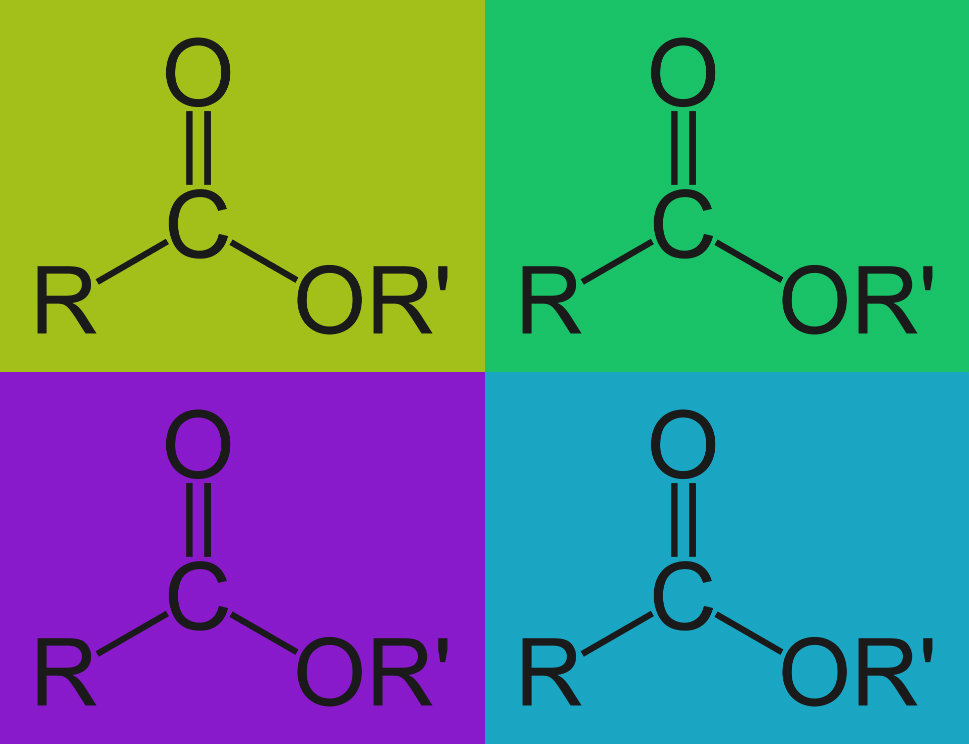
Advanced Cleaning Mechanisms: Alkaline Hydrolysis
Alkaline Hydrolysis is a chemical reaction that takes place when tough organic residues are soaked in an alkaline solutions like detergent. Organic and biological compounds are altered and made unstable by alkaline cleaning agents as they undergo a process called alkaline hydrolysis. Alkaline hydrolysis breaks these residues into smaller more water soluble and more easily emulsified molecules.
There are two types of residues, specifically, that are broken down by alkaline detergents…