Remove Endotoxin Residue
Q: Does Alconox detergent remove endotoxin?
A: Detergents like Alconox® Powdered Precision Cleaner and Liquinox® Critical Cleaning Liquid Detergent, and others, have been used for decades around the globe to create surfaces devoid of residues. A detergent cleaning is a vital part of a depyrogenation program.
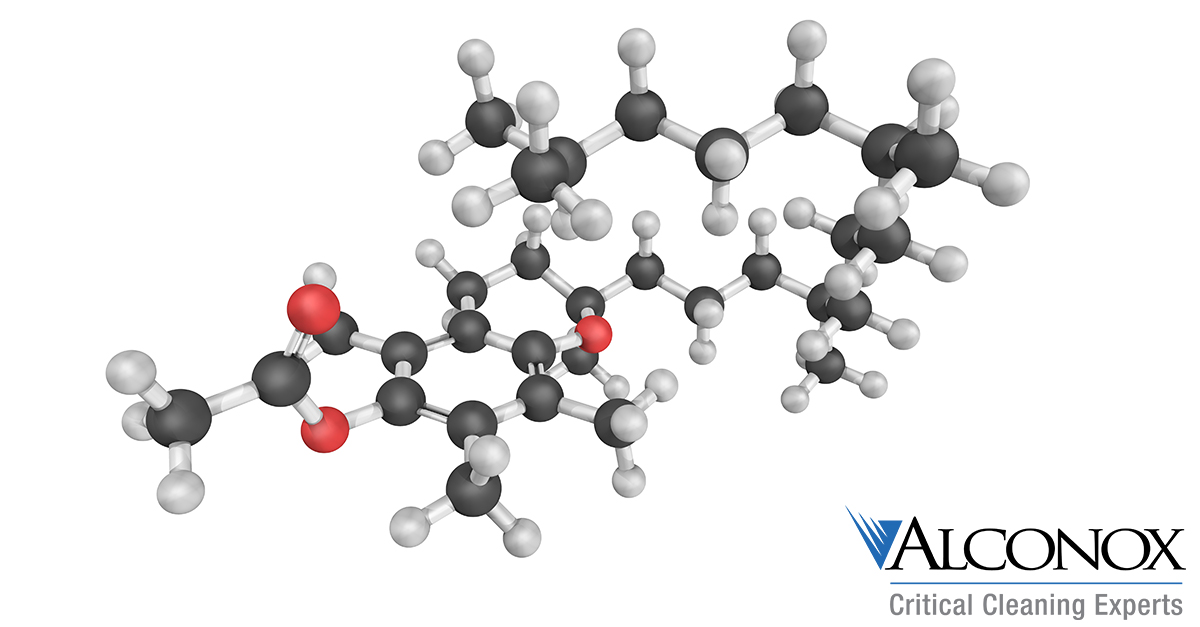
Vitamin E Cleaning
Q: You recently recommended hot water for cleaning cosmetics grade silicone that we were having a big problem with. That seemed to be the missing peace. We have the same struggles with Vitamin E Acetate. Should we use hot there?
A: Vitamin E acetate, or alpha-Tocopherol acetate, can definitely be a cleaning challenge. And indeed heat would be my initial recommendation.
Undissolved Washer Detergent Sign of Bad Seal?
Q: We are having issues with undissolved detergent that is persisting. We make sure the compartment is dry before use. Can we try a liquid?
A: It sounds like you have a cup in door washer for the powdered detergent. The seals there are not designed to hold a liquid – of course some seals are better than others, but they are more for powders and gels (think home dishwasher gel at home).
Specific Formulations for Cold Water Cleaning?
Q: I’m looking for detergents similar to Liquinox and Citranox, that are specially formulated for cold water cleaning conditions. Can you recommend analogs to the two?
A: For most residues, the detergency of an aqueous detergent is enhanced and hastened by heat.
Bleach and Detojet?
Q: We have a washer that use both Detojet and a bleach cycle. We would like to optimize. Can we combine? We have read the “Bleach with Detergents for Disinfection?” article. A: A key component of Detojet® Low Foaming Liquid Detergent is indeed sodium hypochlorite (per the SDS 2.5-10%).