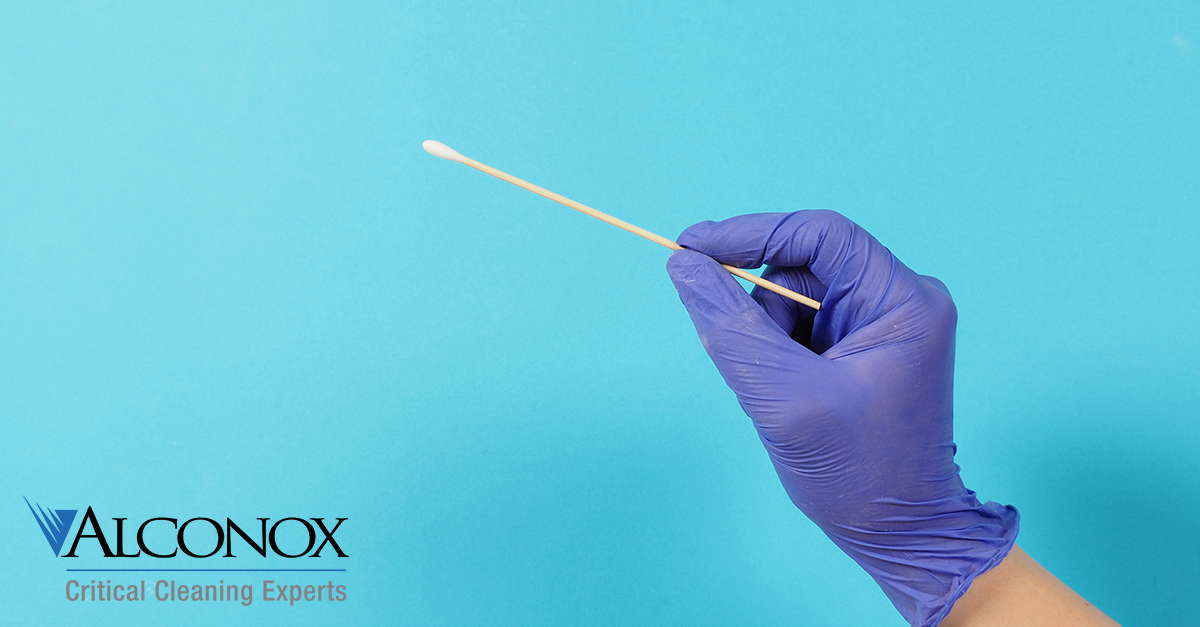
Residue Detection: Swab Method
Q. Do you have a recommended swab detection method for residual detergent?
A. We most certainly do. It is suggested to use a swabbing area of 5 cm X 5 cm. Where extreme low level acceptance criteria on a low recovery residue, i.e. some product residues, it can help to….
Detergent Color Variations
Q. The color of our Liquinox is slightly different by lot number. Why is this happening? Is there an issue, perhaps we have done something wrong with storage?
A. Do not fear! These color variations are normal. There is a range of acceptable color differences in Liquinox detergent. Differences in the age and storage conditions of the raw materials and of the Liquinox detergent itself will account for differences in color
Cleaning Iron Residue in Lab Glassware (Fe+2 and Fe+3)
Q. Please provide advice on cleaning iron residues, Fe+2 and Fe+3, in lab glassware. We need to clean down to sub ppm levels.
A. The best iron salt removing detergent for manual cleaning of lab glassware is Citranox®. Citranox is a mild acid, high chelating detergent for high performance removal of…
9 Steps to Successful Critical Cleaning: BATHOCARD
Q. Did you know there are nine different factors that determine successful cleaning? A. “BATHOCARD” a phrase coined by Alconox technical team.
High Alkaline Detergent on Steel?
Q. Are there ways to remove oxidation on stainless steel without acidic detergents? We are looking to simplify our process and avoid low pH. A. While citric acid based oxidation removal and passivation is our preferred method due to its safety profile, there are ways to remove oxidation from steel with alkaline detergents.