Advanced Cleaning Mechanisms: Alconox Powder in an Auto Dispenser
Q: Looking to implement an auto dispenser for our Alconox, and it is a little bit of a struggle. Are you aware of any test equipment, or alternative methods of measuring which may be able to continuously measure the pH/conductivity at…
How To Choose a Phosphate-Free Clean In Place (CIP) Detergent
We are a biotech company looking to upgrade our cleaning with phosphate-free detergents used in CIP systems. We were recommended to contact Alconox, LLC Can you describe the uses of your low foam detergents, like Solujet, Tergajet and Detergent 8? Any others? What surfactants and cleaning constituents are there?
Cleaning Validation Virtual Summit, 2021
February 10th and 11th….Come join Michael Moussourakis, Senior Director, Alconox, LLC and several other industry experts at the PharmaEd Resources Inc. Cleaning Validation Virtual Summit, 2021.
Getting a Certificate of Analysis for an Alconox, LLC Detergent
Alconox, LLC cleaners that are used to clean pharmaceutical, biotech, medical, food and other regulated industries product contact surfaces, in good manufacturing practice (GMP) validated environments, need certificates of analysis obtained and kept on file. These certificates of analysis (COA) can document the analysis of a specific lot of detergent and verify that it conforms to the manufacturer’s specifications. Confirming that the detergent has not changed.
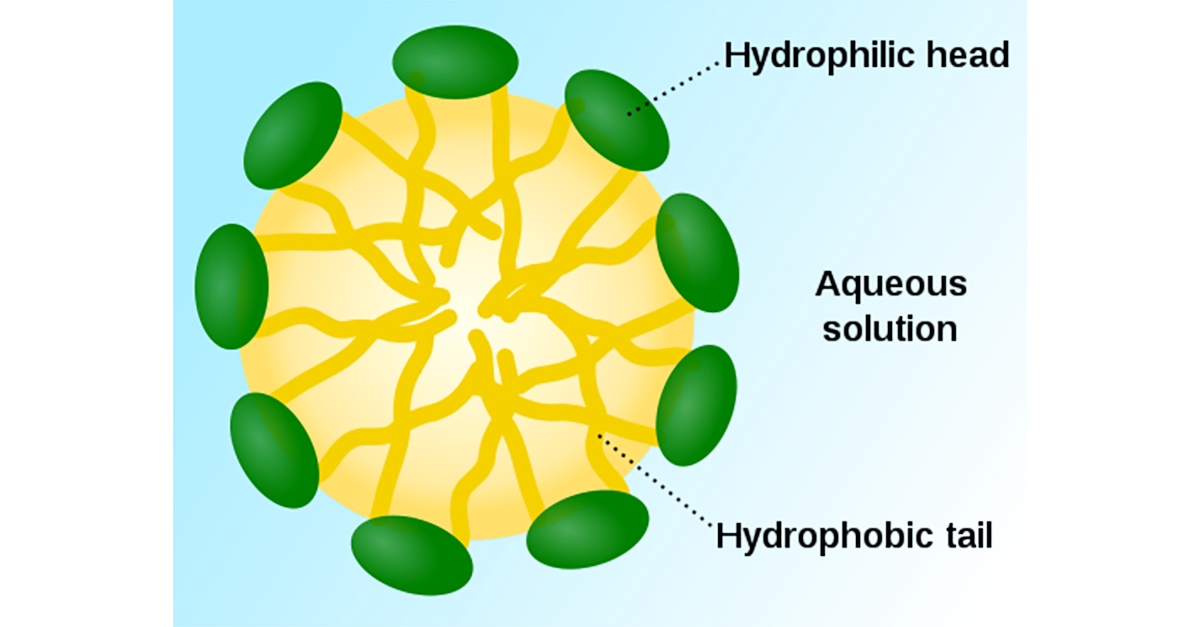
Advanced Cleaning Mechanisms: The Critical Micelle Concentration of Detergent
The critical micelle concentration is the minimum concentration at which the emulsifiers in a detergent will coalesce in to membrane structures of globes, rods or sheets with their hydrophobic (water hating) ends on the inside of the membrane and their hydrophilic (water loving) ends on the outside such that hydrophobic oily residues can be emulsified inside these membrane structures. Micelles are further elaborated on below. If you are below the critical micelle concentration, you do not have effective emulsifying of oily residues.